PSA is your voice in the 7th Community Pharmacy Agreement
The 7th Community Pharmacy Agreement, which commenced on 1 July 2020, provides certainty, stability and flexibility for the pharmacy profession for the next five years. PSA is committed to working with pharmacists to improve medicine safety and to realise their full potential as part of the healthcare team.
19/10/2020: More information on the MRFF grant opportunity is now available. Read more here.
LATEST UPDATES
23 September 2021
- Grace period extended for CTG PBS Co-payment Program. Find out more here
12 July 2021
- Grace period implemented for CTG PBS Co-payment Program. Find out more here
1 July 2021
- The Indigenous Dose Administration Aids (IDAA) Program commences today. Find out more here
- The Indigenous Health Services Pharmacy Support (IHSPS) Program commences today. Find out more here
- Reforms to the CTG PBS Co-Payment Program commence today. Find out more here
In the 7th Community Pharmacy Agreement, $1.2 billion has been allocated to funding patient-focused professional programs. These programs aim to support access to medicines, minimise adverse events and support medication compliance and the quality use of medicines.
The PSA’s Standards and Guidelines for 7CPA professional programs outline best practice for pharmacists delivering these services in their daily practice.
These guidelines do not replace the need for pharmacists to exercise professional discretion and judgment when delivering these programs in their own unique practice environment. These Guidelines do not include clinical information or detailed legislative requirements. At all times, pharmacists delivering these programs must comply with all relevant Commonwealth, State and Territory legislation, as well as to the overarching and program-specific standards, codes, and rules.
Under the 7CPA pharmacists may be eligible to receive payment for providing professional pharmacy programs. For full details on the program rules, eligibility, claiming and payments, please refer to the Pharmacy Program Administrator website www.ppaonline.com.au.
MedsChecks and Diabetes MedsChecks are patient-centred, clinical services delivered with the aim of improving medicine safety and the safe and quality use of medicines. They integrate with other patient-centred services to enhance a patient’s optimal use of medicines. Pharmacists can provide these services to any patient based on their clinical need.
The MedsCheck and Diabetes MedsCheck service involves a review of a patient’s medicines, a face-to-face consultation between the pharmacist and the patient, and the development of a medication profile and an action plan. It focuses on education and self-management and aims to identify medication-related problems (e.g. non-adherence), improve effective use of medicines and provide education about medicines, including their best use and storage.
The delivery of these services encourages pharmacists to work collaboratively with the patient and their carer, prescriber, and other relevant members of the healthcare team to enhance patient care.
Telehealth medication reviews
In response to the COVID-19 pandemic, telehealth services may temporarily be offered where a patient meets the eligibility criteria. The Department of Health has partnered with PSA in developing resources for pharmacists conducting medication reviews via telehealth. These resources are designed to support pharmacists conducting service via telehealth, and to assist patients receiving these service.
For full patient eligibility criteria, refer to the MedsCheck and Diabetes MedsCheck program rules which can be found on the Pharmacy Programs Administrator website www.ppaonline.com.au.
Access the resources below:
The Dose Administration Aid (DAA) service is a patient-centred, clinical services delivered with the aim of improving medicine safety and the safe and quality use of medicines. It integrates with other patient-centred services to enhance a patient’s optimal use of medicines. Pharmacists can provide this service to any patient based on their clinical need.
A DAA service aims to promote medicine safety and the quality use of medicines by improving adherence and medication management, and reducing medication misadventure. A DAA is a device that can be used as part of a coordinated approach to medication management. By using a DAA, patients can receive the correct medicine, at the correct dose and time, in a safe and hygienic manner.
The delivery of the DAA services encourages pharmacists to work collaboratively with the patient and their carer, prescriber, and other relevant members of the healthcare team to enhance patient care.
Comprehensive medication management reviews aim to identify, resolve and prevent medication-related problems, and optimise medicines use in partnership with medical practitioners and patients. This process occurs in consultation with the patient, and in collaboration with a medical practitioner and other healthcare providers, with the aim of optimising each patient’s medication experience and clinical outcomes.
A comprehensive medication management review includes an individualised medication management plan that details the intended goals of therapy. Appropriate follow-up occurs to determine actual patient outcomes.
Comprehensive medication management reviews must be conducted by a pharmacist with the appropriate skills and expertise, acting in partnership with patients as part of a multidisciplinary team. These guidelines apply to comprehensive medication management reviews regardless of practice setting and funding mechanisms.
Telehealth medication reviews
In response to the COVID-19 pandemic, telehealth services may temporarily be offered where a patient meets the eligibility criteria. The Department of Health has partnered with PSA in developing resources for pharmacists conducting medication reviews via telehealth. These resources are designed to support pharmacists conducting service via telehealth, and to assist patients receiving these service.
For full patient eligibility criteria, refer to the Home Medicines Review and Residential Medication Management Review program rules which can be found on the Pharmacy Programs Administrator website www.ppaonline.com.au.
Access the resources below:
Medication review fact sheets
Quality Use of Medicines (QUM) services are a key strategy to optimise medication management within residential aged care facilities (RACFs). They improve medicine safety by supporting RACFs’ to safely manage medicines, and improve medicine management practices and procedures. QUM services revolve around three groups of activities:
- education and training;
- clinical governance; and
- resident-level activities.
These should be implemented following the development of a QUM plan, which integrates these activities into a cohesive quality improvement process.
These guidelines are designed to promote a consistently high quality of service, and provide guidance to pharmacists on professional issues relating to the various activities undertaken within the scope of QUM services.
The staged supply service is a patient-centred, clinical services delivered with the aim of improving medicine safety and the safe and quality use of medicines. It integrates with other patient-centred services to enhance a patient’s optimal use of medicines. Pharmacists can provide this service to any patient based on their clinical need.
Staged supply is a clinically-indicated, structured pharmacist service involving the supply of medicine to a patient in periodic instalments as requested by the prescriber or carer. These instalments are less than the originally prescribed quantity at agreed intervals (e.g. daily or weekly), with the balance of the prescribed quantity is held by the pharmacy to fulfil subsequent instalments.
The delivery of the staged supply service encourages pharmacists to work collaboratively with the patient and their carer, prescriber, and other relevant members of the healthcare team to enhance patient care.
Under Part 2 of the 7th Community Pharmacy Agreement, PSA is recognised as the national peak body for pharmacists in Australia, and the custodian of standards and guidelines governing the professional practice of pharmacists in Australia.
PSA is committed to supporting the highest standard of professionalism in the pharmacy profession in order to support patient access to primary health care and the safe and quality use of medicines.
As identified in Pharmacists in 2023, PSA seeks to identify and unlock opportunities that realise the full potential of pharmacist practice as part of the healthcare team.
The National Competency Standards for Pharmacists in Australia (the ‘competency standards’) describe the individual practitioner’s skills, attitudes and attributes (e.g. values and beliefs) based on knowledge (gained through study) and experience (gained through practice) that together is considered sufficient to enable the individual to practise as a pharmacist. Continuing Professional Development (CPD) and practice improvement programs are designed to help pharmacists identify and develop areas where improvements in their competence is needed. PSA is the custodian of the framework on behalf of the profession.
The National Competency Standards Framework undergoes periodic review to ensure it is consistent with the professional requirements of contemporary pharmacist practice, and reflects the expectations of Australian healthcare consumers.
Continued Dispensing is the supply of an eligible medicine to a patient under the Pharmaceutical Benefits Scheme when there is an immediate need for that medicine but it is not practicable to obtain a prescription, provided:
- the medicine has been previously prescribed, therapy is stable and there has been prior clinical review by the prescriber that supports continuation of the medicine; and
- the medicine is safe and appropriate for the patient.
The Guidelines for Continued Dispensing of Eligible Prescribed Medicines by Pharmacists are intended to provide advice and guidance to assist pharmacists to meet their professional responsibilities, exercise professional judgement in individual circumstances and manage risks associated with the Continued Dispensing of eligible prescribed medicines.
An addendum to the Continued Dispensing guidelines was updated and published most recently on 1 July 2020 with expanded arrangements to support people affected by the COVID-19 Pandemic. The addendum can be accessed here.
PSA has a couple of member-only support tools to assist pharmacists with the Continued Dispensing supply arrangements, including:
In Australia, pharmacists have been administering vaccinations since 2014. It is now widely accepted as being within scope of practice for appropriately trained pharmacists across all states and territories.
The Practice Guidelines for the Provision of Immunisation Services provide information and guidance to pharmacists on professional issues and obligations relating to pharmacist immunisation services. These guidelines may also be a useful resource for other stakeholders, including service providers or immunisers contracted or employed to administer vaccinations as part of a pharmacy immunisation service.
PSA recognises the importance of continuity of care within the healthcare environment. These guidelines promote specific policies and protocols designed to ensure safe and effective service delivery and communication between healthcare providers.
The Guidelines for pharmacists administering medicines by injection provide information and guidance to pharmacists on professional issues and obligations relating to pharmacists administering medicines by injection.
If you are a pharmacist immuniser, you can build your confidence and competence in administering medicines by injection, by completing PSA Administering medicines by injection course.
PSA recognises the importance of continuity of care within the healthcare environment. These guidelines promote specific policies and protocols designed to ensure safe and effective service delivery and communication between healthcare providers.
PSA is committed to promoting quality use of medicines (QUM) in Aboriginal and Torres Strait Islander communities. PSA recognises the need to improve the awareness and understanding of Aboriginal and Torres Strait Islander health and cultural issues amongst pharmacists and pharmacy staff. PSA encourages pharmacists to develop relationships with Aboriginal and Torres Strait Islander people and communities in their local area to optimise health benefits to community members.
The Guide to Providing Pharmacy Services to Aboriginal and Torres Strait Islander people is designed to assist pharmacists to deliver a consistently high quality of service to Aboriginal and Torres Strait Islander people, to communicate effectively and to be culturally responsive health professionals. The guide encourages increased engagement with Aboriginal health services (AHSs), key Aboriginal organisations and Aboriginal and Torres Strait Islander people.
The Digital Health Guidelines for Pharmacists describe the professional obligations of pharmacists when interacting with digital health systems in performing their professional clinical roles.
These guidelines provide guidance on expected professional practice when using digital health systems so that pharmacists can provide optimal patient outcomes while meeting legal and ethical obligations relating to data access and stewardship.
The guidelines should be used as a tool to support practice and professional decision making, and ensure that patients’ needs, beliefs and preferences are met. They can be used as an educational resource to inform quality assurance processes and provide support when resolving legal disputes and ethical dilemmas.
Dispensing is a fundamental component of pharmacy practice, facilitating the safe provision of prescription medicines.
The Dispensing Practice Guidelines provide information and guidance to assist pharmacists to meet their professional responsibilities, exercise professional judgement and manage risks associated with dispensing. They provide information and advice to pharmacists on professional issues related to dispensing. The guidelines highlight contemporary considerations for pharmacists from the point of receiving a prescription, including:
- reviewing prescribed medicines effectively using the breadth of information sources available
- recording and documenting information to support decision making, taking into account the need to protect patient information
- selecting or accurately preparing the medicine
- labelling
- counselling.
Clinical governance is a concept which has been recognised as a way of achieving and improving safety, quality and effectiveness in the provision of health care. While all pharmacy services contain a degree of quality management and governance, the formal application of clinical governance to services led by pharmacists is highly varied.
Clinical Governance Principles for Pharmacy Services considers clinical governance concepts to provide pharmacists and organisations involved in the provision of pharmacy services with guiding principles for the design, implementation and ongoing evaluation of pharmacy services.
The principles can ideally be used by service designers, organisations, and individual pharmacists to help plan new pharmacy services, or reflect on opportunities to enhance aspects of clinical governance to improve the quality of patient care for existing pharmacy services.
6CPA Pharmacy Trial Program Guidelines
Pharmacy trials that were established and funded under the 6th Community Pharmacy Agrement and that are intended to continue beyond the term of that agreement will continue until the current trial end date.
Health Care Homes is a collaborative model of care for patients with complex and chronic needs to improve their disease-state control. The patient is managed at a home base, known as a Health Care Home, allowing the delivery of coordinated, team-based care around the needs and goals of the patient. Health Care Homes are existing general practices or Aboriginal Community Controlled Health Services. The model uses a Shared Care Plan to communicate the patient’s mutually agreed health information with the patient and all members of their healthcare team.
The Guidelines for Pharmacists Participating in the Health Care Homes Trial guidelines provide pharmacists with best-practice guidance for the delivery of the Community Pharmacy in Health Care Homes Trial Program (the Trial Program).
PSA Member-Only Content
Certificate for absence from work is a form of evidence used to confirm that an employee is unable to attend work either due to illness, injury, or having to care for family or a household member. Other forms of evidence are medical certificates and statutory declarations. It is deemed within a registered pharmacist’s scope of practice to issue a certificate for absence from work, provided they are acting within their competency and professional expertise as a pharmacist.
The Absence from Work Certificate: Guidelines for Pharmacists provide pharmacists with best practice guidance on issuing certificates for absence from work. This Guidance has been developed to meet the Fair Work Act 2009 requirements on evidence to support the need for an employee to be absent form the workplace. The Guidelines do not replace the need for pharmacists to exercise professional discretion and judgement when issuing a certificate for absence from work.
The PSA recognises the importance of continuity of care within the healthcare environment. The Practice Guidelines for the Provision of Sleep Apnoea Services Within Pharmacy provide assistance to pharmacist on professional issues and obligations relating to pharmacy sleep apnoea services and aim to promote standardisation of this professional service within pharmacies in Australia.
They also promote policies and protocols designed to ensure effective channels of communication and collaboration between healthcare providers to ensure best possible outcomes for the patient.
The PSA, developed these practice guidelines in conjunction with the Australasian Sleep Association. This work is the result of extensive consultation and collaboration between the two organisations over some time.
CODE
OF ETHICS
The PSA’s Code of Ethics underpins the professional practice of all pharmacists in Australia
Access the Code of Ethics below
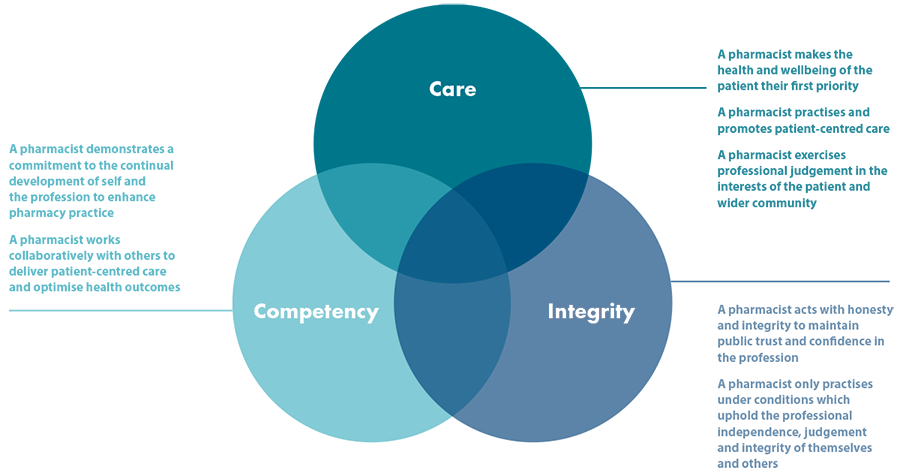
The PSA’s Code of Ethics for Pharmacists (the ‘Code’) articulates the values of the pharmacy profession and expected standards of ethical behaviour of pharmacists towards individuals, the community and society. The Code underpins the professional practice of all pharmacists in Australia.
The Code undergoes periodic review to ensure it is consistent with the ethical requirements of contemporary pharmacist practice and reflects the expectations of Australian healthcare consumers. It comprises seven principles aligned to three ethical values pertinent to professionalism: Care, Integrity and Competency. Each principle is supported by a number of obligations that articulate the behaviours that encompass these values in pharmacy practice.
PROFESSIONAL
PRACTICE
STANDARDS
The PSA’s Professional Practice Standards underpins the professional practice of all pharmacists in Australia
Access the Professional Practice Standards below
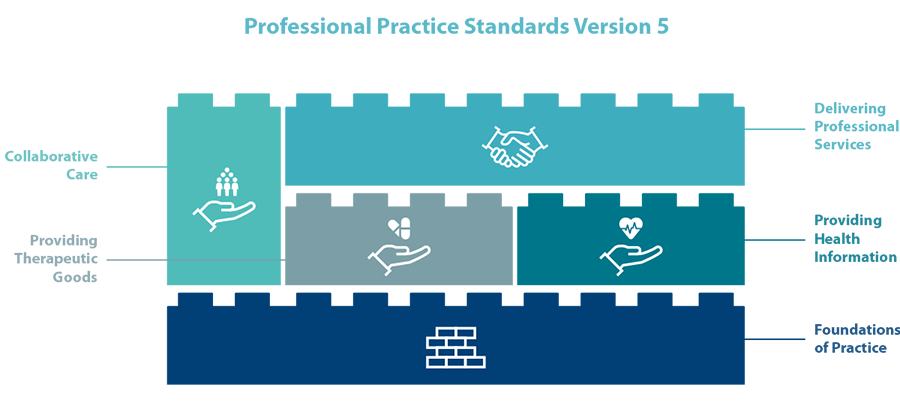
PSA’s Professional Practice Standards articulate the values of the pharmacy profession and expected standards of professional behaviour of pharmacists towards individuals, the community and society. The Professional Practice Standards underpins the professional practice of all pharmacists in Australia.
The 16 Standards are structured into key streams to clearly articulate the roles and activities that pharmacists undertake in contemporary professional practice:
- Foundations of Practice
- Providing Therapeutic Goods
- Providing Health Information
- Delivering Professional Services
- Collaborative Care.
OTHER
MEASURES
PSA is supporting a number of additional measures outside of the agreement
Medical Research Future Fund
On 25 September 2020, the Australian Government announced the investment of $25 million into research to improve the safe use of medicines and pharmacist-led interventions through the Medical Research Future Fund (MRFF).
Federal Minister for Health, Greg Hunt, announced the new Quality, Safety and Effectiveness of Medicine Use Intervention by Pharmacists MRFF Grant Opportunity, designed to support Medicines Safety as Australia’s 10th National Health Priority.
2022 Grant Opportunity – Quality, Safety and Effectiveness of Medicines Use and Medicine Intervention by Pharmacists
This grant opportunity will open on Thursday, 16 June 2022 and closes on Wednesday, 7 September 2022.
The 2022 Quality, Safety and Effectiveness of Medicine Use and Medicine Intervention by Pharmacists grant opportunity is part of the MRFF and the Preventive and Public Health Research Initiative. It provides researchers with funding to improve the safe and effective use of medicines.
In 2022, grants of up to $1.5 million will be available under four Streams to support eligible organisations to undertake research to support innovative approaches to improve the quality and cost‑effectiveness of preventive healthcare interventions.
The objective of the program is to provide grants to Australian medical research and medical innovation projects that align with the following streams:
- Stream 1: assess the feasibility (including cost effectiveness, and acceptability by patients and prescribing health professionals) of pharmacogenomic interventions by community pharmacists designed to optimise medication prescription and use.
- Stream 2: assess the feasibility (including cost effectiveness, scalability, and acceptability by patients and prescribing health professionals) of pharmacogenomic interventions to optimise the prescription and use of medicines for treating patients with cancer (oncology and supportive care).
- Stream 3: implement, validate, and cost approaches that involve community and hospital pharmacists collaborating to improve medication management at transition into the community.
- Stream 4: implement, validate, and cost approaches for pharmacist interventions in residential aged care using objective patient focused indicators.
The outcomes of the research funded by this grant is to improve the health and wellbeing of Australians by supporting the safe and effective use of prescription medicines, quality of care provided to patients, reducing hospital readmissions due to medication complications and evidence to support the safe and effective use of medicines in residential aged care.
More information is available here. Key documents, including the Grant opportunity guidelines, are available here.
Contact us using the form below to find out how PSA can support you with your 2022 grant application.









































